IDPH is first step in Zika testing

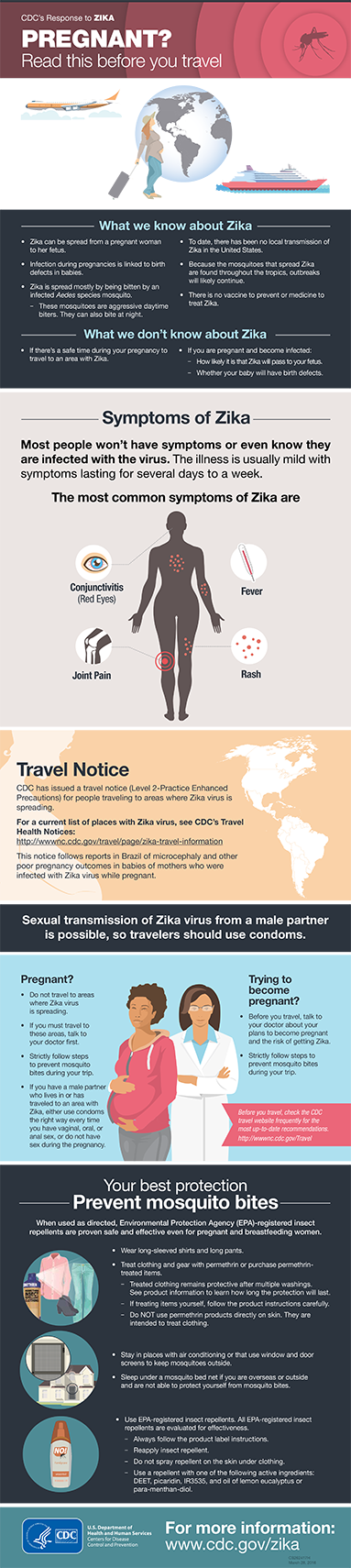
Zika virus testing should be considered for patients with acute fever, rash, myalgia, or arthralgia who have traveled within the previous two weeks to an area with ongoing transmission of those viruses or are living in an area with ongoing transmission.
During the first week after onset of symptoms, Zika virus disease can often be diagnosed by performing reverse transcriptase-polymerase chain reaction (RT-PCR) on serum. Virus-specific IgM and neutralizing antibodies typically develop toward the end of the first week of illness; cross-reaction with related flaviviruses (e.g., dengue and yellow fever viruses) is common and may be difficult to discern. Plaque-reduction neutralization testing can be performed to measure virus-specific neutralizing antibodies and discriminate between cross-reacting antibodies in primary flavivirus infections.
CDC recommends serologic testing for asymptomatic pregnant women with a history of travel (2 to 12 weeks post travel) to areas with local transmission of Zika virus or who are living in an area with ongoing transmission.
The most recent information concerning affected countries, symptoms, treatment, etc. can be found on the CDC Zika web page.
The following information is for Iowa clinical and hospital laboratories.Diagnostic Testing:
- Healthcare providers suspecting a potential case of Zika virus should FIRST contact the Iowa Department of Public Health’s Center for Acute Disease Epidemiology at 800-362-2736. CADE staff will consult with the provider to determine whether the case meets the CDC testing criteria.
- If testing is warranted, CADE staff will determine the specimens to collect, send out a pre-populated CDC specimen submission form (CDC 50-34), and make arrangements for the courier to pick up collected specimens and deliver them to the State Hygienic Laboratory.
- All specimens should be submitted to the Hygienic Laboratory with a completed Viral and Bacterial PCR and DFA Test Request form. If not already listed, indicate the specimen type on the “Other” line in the Specimen Information section.
- Write in Zika testing under the tests requested section and the recommended tests to be performed. Complete the pre-populated CDC specimen submission form CDC 50-34 to send along with the specimen(s). A specimen should be triple packaged as a Category B Biological Substance.
The specific tests performed will depend on the timing of the specimens relative to illness onset and clinical information as outlined in the testing algorithm.
Please provide the date of illness onset, dates of specimen collection, specimen type, description of clinical illness, travel history, flavivirus vaccination history, and contact information for the submitter. Testing primarily will be performed on serum or CSF.
Zika Virus Specimen Collection and Submission Instructions:
AdultsSerum- at least 1.0 mL send cold on ice packs or frozen.
IF CSF is already collected and available, send 0.5mL frozen.
InfantsSerum-at least 1.0 mL, send cold on ice packs or frozen.
Amniotic fluid or cord blood -at least 1.0 mL, send frozen.
Placenta- portion in sterile container, send frozen.
Note: Samples that are to be sent frozen should be frozen as immediately as possible after collection.Laboratory safety:
Zika virus is classified as a biological safety level (BSL) 2 pathogen. It should be handled in accordance with Biosafety in Microbiological and Biomedical Laboratories (BMBL) guidelines and a laboratory risk assessment should be performed for the specific procedures utilized for testing specimens from patients suspected of carrying the virus.
Results:Test turn-around time is expected to be within 7 days. There may be delays if confirmatory testing is required.
ALL RESULTS WILL BE SENT TO THE APPROPRIATE STATE OR LOCAL HEALTH DEPARTMENT.
Reporting:Zika, dengue and chikungunya are all nationally notifiable conditions. Health care providers are encouraged to report suspected cases to their state or local health department to facilitate diagnosis and mitigate the risk of local transmission.
Resources:- For technical questions for SHL, contact:
- Dr. Wade Aldous (319) 335-4765;
- Jeff Benfer, Molecular Biology Supervisor (319) 335-4276; or
- Michelle Sexton, Maternal Screening Supervisor (319) 335-4434
The toll-free number for these contacts is 800-421-IOWA (4692).
- Addition information about the Zika virus is available online
- Centers for Disease Control and Prevention
- Pan American Health Organization